Introduction:
Lymphoplasmacytic lymphoma (LPL) is as an indolent neoplasm of B-lymphocytes with plasmacytic differentiation causing bone marrow infiltration along with variable involvement of lymph nodes/or spleen. Most common subtype of LPL, Waldenstrom Macroglobulinemia (WM) involves IgM monoclonal gammopathy of any concentration. Due to widespread availability of targeted therapy drugs (BTKi), the treatment landscape of LPL is changing. The aim of this study is to analyze disease characteristics, patterns of real-world treatment selection and its impact on clinical outcomes (Objective response rate (ORR), Progression free survival (PFS) and Overall survival (OS).
Methods:
A retrospective analysis of patients (age ≥18 years) with diagnosis of LPL/WM between January 1, 2003, to March 31, 2023, seen in consultation at Roswell Park Comprehensive Cancer Center was performed. ORR (CR, VGPR, PR and MR) was calculated according to response criteria at 6 th International Workshop for WM. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). OS and PFS were reported using standard Kaplan-Meier methods. Multivariate cox regression modeling was used to assess associations between first line regimen and survival outcomes (OS / PFS). Predictor variables, namely age at diagnosis, gender, Charlson Comorbidity Index (CCI) score, and rIPSS (revised International Prognostic Score System) were included in multivariable models.
Results:
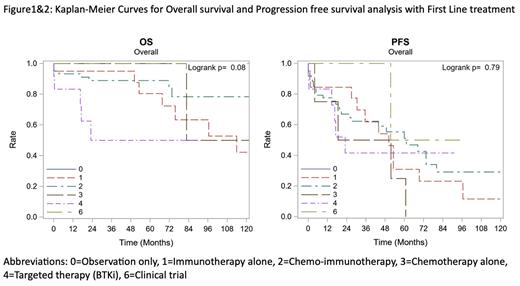
A total of 127 patients were included in this study. The median age at diagnosis was 68 years (range 41 to 88), of which 75 (59.1%) were men. Majority of the population was White (n=112, 88.2%) and had ECOG of 0 (n=70, 67.3%). Monoclonal gammopathy of IgM subtype was present in 114 (94.2%), IgG in 3 (2.5%) and IgA in 1 (0.8%) patient. MYD88 mutation testing was positive in 69.8%, negative in 14% and unknown in 16.3% patients. Two out of 10 patients tested for CXCR4 mutation were positive. Sixteen (28.1%) patients had hyperviscosity symptoms and underwent plasmapheresis with first line treatment. These patients had a mean IgM level of 6249.5 mg/dL and mean M-spike of 4.53 g/dL. Of 121 patients with data on treatment strategy, 20 (16.5%) were monitored with observation alone. The most common treatment modality used in first line was chemo-immunotherapy (C-I) in 61 (50.4%) patients followed by Rituximab immunotherapy (R-I) in 20 (16.5%) patients and BTK inhibitors in 12 (9.9%) patients. ORR was highest 90.7% (39/43) in chemoimmunotherapy group, 87.5% (7/8) in BTKi group and 71.4% (10/14) in Immunotherapy group. Bendamustine-Rituximab was the most common used treatment regimen (n=42), with resultant CR in 20% and VGPR in 23.3% patients. At median follow-up duration of 36.9 months, median OS was 113.9 months in R-I, 159.8 months in C-I and not reached (NR) in BTKi group. The median PFS was 50.2 months in R-I, 61.2 in C-I and 23 months in BTKi group. Although Multivariate Cox Regression analysis failed to show statistically significant difference in PFS, there is tendency towards improved OS in C-I group (p=0.08) among all the groups when adjusted for age, sex, CCI.
Conclusion:
In our single center experience, chemoimmunotherapy combination with Bendamustine-Rituximab is the most used treatment choice in first line settings followed by single agent anti-CD20 therapy and BTKi. Although Multivariate Cox Regression analysis failed to show statistically significant difference in PFS, there is a tendency towards improved OS in the chemoimmunotherapy subgroup (p=0.08). The shorter PFS in BTKi group is likely due to toxicity of targeted therapy leading to treatment interruption and change of therapy.
Disclosures
Hernandez-Ilizaliturri:AbbVie: Consultancy; Dava Oncology: Consultancy; Gilead: Consultancy; Incyte/Morphosys: Consultancy; Novartis: Consultancy; ADC Therapeutics: Consultancy; Epizyme: Consultancy; Collectar: Consultancy; BioGene: Consultancy; Amgen: Consultancy; BMS: Consultancy; Kite: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal